Explosion risks related to Ammonium Nitrate
9th September 2021
What is Ammonium Nitrate (AN)?
AN is used as a fertiliser in the agriculture industry. Another major use is as a component of industrial explosive mixtures and blasting agents used in mining, quarrying and civil construction. Liquid AN (AN dissolved in water) may be sold as fertilizer. Solid AN is produced in the form of prills (beads), grains, granules, or crystals. High density prills, granules and crystals are used as fertilisers. Grains and low density prills are predominantly used in the production of explosives and blasting agents.
AN melts at 170°C and begins to undergo decomposition at 210°C. Once it decomposes, energy releases which causes the temperature to rise even further especially when the AN is stored in a confined space where heat is not vented. The higher the temperature, the more vigorous the decomposition reaction. Decomposing AN can reach very high temperatures, when these exceed 1000°C, the decomposition reaction becomes explosive.
Generally, AN that contains <0.2 % combustible substances and AN fertilisers are classified as an oxidizer. AN itself is not combustible. Being an oxidizer, AN can assist other materials to burn or accelerates burning.
The most credible explosion hazard in AN storage is a neighbouring fire leading to an explosion. The explosion risk increases when AN is stored indoors in unventilated places and when stored with other combustible materials. The aforementioned is what happened in Beirut.
There is a common misconception that AN is highly explosive leading to imposing excessive and sometimes wrong measures.
Misconceptions around AN
Because of the Beirut incident and because of other incidents that have happened in the past, AN is often wrongly considered as an explosive which is not the case. The fact that AN could explode does not make it an explosive. In order to clarify this, we have to compare AN with other products such as Ammonium Nitrate Fuel Oil (ANFO) which is basically 94.5 % AN mixed with 5.5% diesel fuel. ANFO is categorised as a blasting agent. Also a blasting agent is not an explosive. It needs a significant ignition source in order to explode. Detonators are used as an ignition source to cause blasting of blasting agents. A typical detonator is Pentaerythritol Tetranitrate (PETN). PETN is typically manufactured in powder form and is put in blasting caps in amounts of less than 1 gramme. As can be seen in the following picture (Examples of blasting caps), the blasting can be triggered by an electrical impulse through the electrical wires connected with the blasting cap.
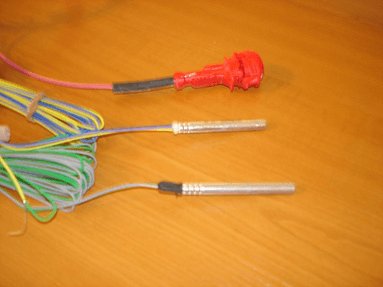
In other words, even ANFO can be handled and transported relatively easily and will not explode easily. Detonators such as PETN will explode when adding low amounts of energy.
Regulations around storage and handling
AN is typically stored in bags of approximately 1 tonne. The picture below (AN storage) shows AN in bags stored outside.

International standards such as NFPA 400 [1]NFPA 400 – Hazardous Materials Code and NFPA 495 [2]NFPA 495 – Explosive Materials Code do provide detailed guidelines. NFPA 400 formulates regulations for indoor storage of AN as briefly illustrated in the following picture (AN storage regulations as per NFPA 400.):
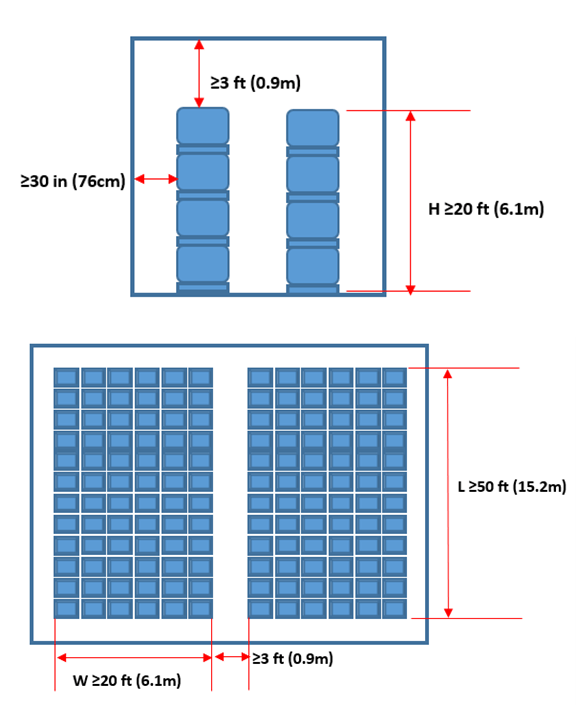
Other widely used guidelines are the International Ammunition Technical Guidelines (IATG). Note that pure AN does not fall under the scope of IATG, ANFO however does.
In general, fire and explosion risks are mitigated by maintaining separation distances and by limiting the amount of product stored in one area. Separation distances are formulated towards inhabited buildings, public roads and other storage or handling buildings. AN is typically stored in bulk of several hundreds of tonnes in warehouses or just outside maintaining rules related to storage height and quantity and separation to other materials. In case of handling and storage in buildings fire safety systems such as sprinklers, smoke detection and smoke or heat ventilation systems are often required. ANFO is typically stored in limited amounts (several tonnes) in igloos or magazines which can withstand overpressures. An example of a typical igloo can be seen in the folloing picture (Typical Igloo construction):

Detonators are stored in normal buildings but in such small quantities, less than half a kilogram, that no major explosions can occur if stored properly.
Beirut Incident in August 2020 – What went wrong
Based on the historical major incidents involving AN, explosions were the result two major classes of incidents:
- Explosion due to an external severe shock
- External Fire spreading into the AN or to a mixture of AN
The Beirut AN Explosion resulted from a fire which broke out in a warehouse in the Port of Beirut where 2,750 tonnes of ammonium nitrate were stored. The AN that had been seized from an abandoned ship in 2014 was impounded and stored for six years in the warehouse storing other materials.
The investigation report published by forensic-architecture.org presented photos of the ammonium nitrate bags using photographs taken in January 2020. Some of the materials reportedly stored inside the warehouse include 23 tonnes of firework, 1000 car tires, Ammonium phosphate, tea & coffee, slow burning detonating cords. Different angles of video footage showed that many windows and doors were closed, which resulted in confinement of the fire building up inside the warehouse. The following non-compliances were noted:
- Improper storage:
- stack arrangement
- AN bags torn open
- AN spillage
- contamination
- presence of combustible materials adjacent to AN stacks
- other explosives
- No appropriate fire protection systems:
- detection system
- suppression systems
- smoke/heat ventilation system
Summary
Due to the Beirut explosion and other explosions that have occurred in the past, there is a general conception that AN is highly explosive leading to imposing excessive and sometimes wrong measures.
Though history has shown that explosions can occur, not only with AN but also with other oxidisers, AN incidents could occur if there are large amounts (hundreds of tonnes) of AN involved in the presence of significant external energy sources; either in the form to heat (fire) or mechanical impact. Confinement and mixing of AN with other substances will contribute to the risk of an explosion occurring.
When stored and handled in the proper manner though, the risk of AN explosions occurring is low. Even in the case of ANFO, which is a blasting agent with AN as a base ingredient, risks of unwanted explosions occurring are minimal when the proper storage and handling practices are maintained.
This publication presents the views, thoughts or opinions of the author and not necessarily those of HKA. Whilst we take every care to ensure the accuracy of this information at the time of publication, the content is not intended to deal with all aspects of the subject referred to, should not be relied upon and does not constitute advice of any kind. This publication is protected by copyright © 2024 HKA Global Ltd.



